We all, for one reason or another, one day cook the brains and think: "Did I caught this sh!t after being with that person?... And if I have it now?..." Let me tell you my personal experience!
I am no exception, and no matter how cautious we can be and strive to stay healthy, there are always doubtful disturbing thoughts now and then. Especially before flying abroad and start thinking: "and if my PCR test result suddenly shows positive and all my travel plans go down the drain?"
That said, I decided to purchase a Rapid Test to detect coronavirus antigens called CITO TEST COVID-19 Ag.
It is not a foolproof guaranteed test, but being 99.5% specific and having a sensitivity of 90.4%, I decided to spend the 500 hrivnas in the pharmacy and relief my brains. Though it is useless to show to the authorities when flying, it is an excellent pacifier for any doubtful mind.
Thank God, so far so good, my test was negative, and as usual, I decided to share with my readers what I know and prove with my personal experience and trustworthy sources. It is not that I know more; it is that I am in Ukraine for longer. (Since 2005)
I hope you enjoy reading and find the information below useful if you are in doubt.
Why not give it a try? It costs $18 and takes 10 minutes to see the result and feel better.
I cared to translate the product specifications and instructions in the package, so you know what to do.

Pharmasco Ltd. is a leading supplier of rapid diagnostic devices for Ukrainian health care institutions, physicians, and patients. Among many other rapid tests from this company, we can find tests to determine rotavirus infection, influenza A and B, HIV, hepatitis A and C, salmonella, and many other diseases.
CITO TEST COVID-19 Ag
Rapid test for coronavirus antigens
TU U 20.5-42579962-014: 2020
INSTRUCTIONS
Catalog number: A502INCPS
APPLICATION
CITO TEST COVID-19 Ag is a rapid test for the qualitative detection of coronavirus antigens (SARS-Cov-2) in nasopharyngeal swabs of persons with suspected coronavirus infection.
IMPORTANT
Please follow the instructions for use to get the correct result. There are many reasons for getting the wrong results.
After testing with the results obtained, you should see a doctor and make the final diagnosis after clinical and additional laboratory tests.
OVERVIEW
COVID-19 (Corona Virus Disease-2019) is an infectious disease with a droplet transmission mechanism caused by the last of the recently discovered coronaviruses (SARS-CoV-2), characterized by predominant upper respiratory tract involvement and general intoxication (patients develop fever, cough, shortness of breath (shortness of breath), sore throat, nasal congestion, malaise, headache, muscle aches, fatigue, weakness, loss of taste and smell (smell), and may lead to the development of SARS, severe acute respiratory syndrome, septic shock, and even death.
COVID-19 can be contracted from a person who is infected with the virus, mainly by inhaling small drops that are released from an infected person's nose or mouth when they cough, sneeze or talk. That is why it is necessary to keep at least 1.5 m (m) away from the sick person. These drops can settle on the surrounding objects and surfaces, such as tables, door handles, and stair railings. As a result, others can become infected with COVID-19 by first touching these objects or surfaces and then touching their eyes, mouth, or nose. Therefore, it is vital to regularly wash your hands with soap and water or treat them with an alcohol antiseptic.
In our country, the incidence of COVID-19 remains very high. According to scientists, the actual number of diseases exceeds official statistics. People of all ages are susceptible to the infection, especially those with weakened immune systems. People over the age of 60, pregnant women, and people with chronic diseases are at risk because they have a very high development of adverse effects of the disease. Complications that develop as a result of this disease pose a severe threat to human health and life. To avoid complications, it is necessary to diagnose COVID-19 in time and seek medical help.
Rapid tests are used as a preliminary screening diagnosis of crown viral infection. These tests can detect IgM or IgG antibodies or specific SARS-Cov-2 antigens. Each test has a different diagnostic value in different periods of the disease. Rapid tests for detecting SARS-Co-2 antigens are used for early preliminary diagnosis of the disease in persons with suspected COVID-19. Typically, coronavirus antigens are detected in samples from the upper respiratory tract in the acute phase of infection within 5-7 d (d) * after the first symptoms of acute respiratory disease. Subsequently, the viral load decreases, and the immune response increases, so from the 2nd week of the illness, it is advisable to conduct tests for antibodies.
It should be noted that the combined use of antigen and antibody tests increases the effectiveness of the diagnosis of coronavirus infection. 1,2 The practicality of using rapid tests for screening diagnosis of coronavirus infection is described in the FDA Guidelines, BOO3 and in the Standards of medical care "CORONAVIRUS DISEASE (COVID-19)" of the Ministry of Health of Ukraine.3, 4, 5
CONTENT:
• Test cassette
• Test tube with buffer
• Sterile swab/applicator
• Disposable gloves
• Instructions
WARNING:
• For self-control.
• Do not use it after the expiration date.
• Do not eat, drink or smoke at the test site.
• Do not use the test if the integrity of the package is damaged.
• Use only the accessories provided with the test for testing.
• The test procedure and instructions must be strictly followed. Correct sampling is critical to perform the test. Failure to follow the knife procedure will result in inaccurate results.
• Samples should be treated as potentially infected material. If an outsider takes the sample, he/she should wear disposable gloves and a protective mask.
• After use, the test and used components should be placed in a plastic bag and disposed of in a waste container.
EXPLANATION OF THE METHOD
Rapid test for detecting coronavirus antigens SITO TEST COVID -19 Ag is a quick qualitative analysis with a visual accounting of test results. During testing, the sample reacts with reagents that have been pre-applied and dried on the test membrane. In the case of a positive result, if the sample contains SARS-Cov-2 antigens, it will form s a color test line. The presence of a control line serves to confirm a sufficient number of samples used, filling the capillaries of the membrane and internal quality control of reagents.
METHOD OF USE
Sample collection
1. Tilt your head back about 45-70°.
2. With a light motion, insert the tip of a sterile swab into the nostril and push it along the outer wall of the nose parallel to the palate to a depth of about 5 cm (cm) for adults and 3 cm (cm) for children until you feel resistance to the back of the pharynx*. (*the membrane-lined cavity behind the nose and mouth)
3. Rotate the swab inside for a few s (c) ** to allow the swab to soak.
4. Slowly rotate to remove the swab from the nasal cavity.

NOTE: Testing should be performed as soon as possible after sampling. If testing is not possible immediately, place the swab with the sample in a dry, sterile, tightly closed test tube. This sample can be stored for 8 hours at room temperature and 24 hours if at 2-8 ° Celsius. (35.6-46.4 °F)
Sample preparation
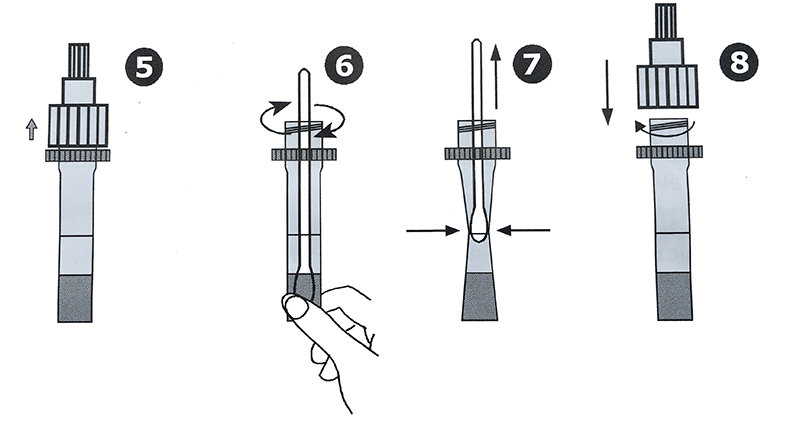
5. Unscrew the lid of the buffer tube.
6. Insert a sterile swab into the buffer tube. Rotate the swab for approximately 10 seconds while pressing the swab against the inside wall of the tube.
7. Remove the swab by squeezing the walls of the tube to get the maximum amount of fluid out of the swab.
8. Close the tube with the lid.
TESTING PROCEDURE
1. Prepare all the necessary materials for testing: a watch, a test cassette, a sterile swab, a test tube with buffer, and a sample. Proceed at room temperature.
2. Open the sealed package, remove the test cassette from the container and use it within 1 hour. You can obtain the best result if you test immediately after opening the box.
3. Place the test cassette on a clean, level surface.
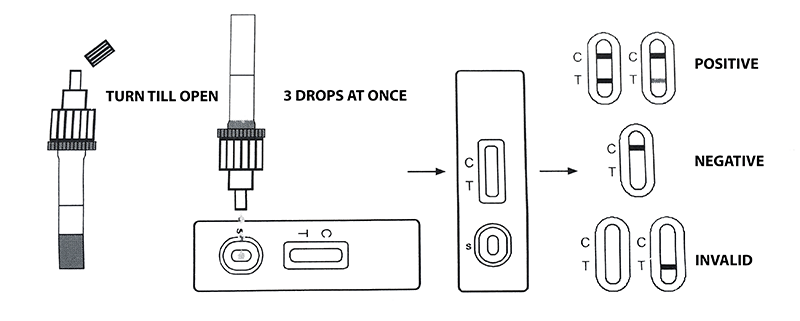
4. Unscrew the top of the sample tube lid, turn it over, and add 3 drops of the resulting mixture to the cassette window marked with the letter (S). Start counting down the time.
5. Record the result in 15 min (min). Do not take into account the test results later than 20 min (min).
ACCOUNTING THE RESULT
POSITIVE: *** two colored lines appear. One colored line should appear in the control area (C) and the other in the test area (T). A positive result indicates the presence of coronavirus antigens in the sample.
***NOTE: the color intensity of the lines in the test area may vary depending on the concentration of coronavirus antigens in the sample. Therefore, soldered colored lines of any intensity in the test area should be considered positive.
NEGATIVE: One colored line appears in the control area (C). There is no line in the test area (T).
INVALID: the control line does not appear. The reason may be the insufficient quantity of the tested sample, non-compliance with the testing procedure, expiration dates, and storage conditions of rapid tests. If an invalid test result is obtained, the test should be repeated using another test cassette.
QUALITY CONTROL:
The test is equipped with internal quality control (forming a color control (C) line). The appearance of a control line indicates a sufficient amount of sample used and compliance with the testing procedure.
SPECIFICATION:
The CITO TEST COVD-19 Ag was determined in clinical trials, having a sensitivity of 90.4% and specificity of 99.5%, respectively.
CONDITIONS OF STORAGE AND TRANSPORTATION
The test can be stored and transported at a temperature of 2-30 °C. The test remains stable until the expiration date indicated on the sealed package. The test should be in a sealed package until use. Do not freeze. Please do not use it after the expiration date. The test has a life term of 24 months.
LIMITATIONS:
1. Rapid CITO TEST COVD-19 Ag should only be used to detect SARS-Cov-2 virus antigens in nasopharyngeal swabs of persons with suspected coronavirus infection. The test cannot determine either the quantitative content or the degree of increase in the concentration of SARS-Cov-2 virus antigens in the sample.
2. A positive test result indicates only the presence of SARS-Cov-2 antigens in the sample and should not be the only criterion for the diagnosis of coronavirus infection. After testing with the results obtained, see a doctor. A physician should make the final clinical diagnosis and additional laboratory tests.
3. If the test result is negative and COVID-19 symptoms are present, it is recommended to repeat it in a few days or perform PCR testing.
4. A negative test result does not eliminate the coronavirus infection, especially when in contact with infected persons, and should consider the possibility of further PCR testing.
5. A negative test result is possible in the presence of coronavirus antigens in the sample at a concentration below the detection limit of the test.
6. Excess blood or mucus in the sample can lead to erroneous results.
LINKS:
1. A preliminary study on serological 1 assay for severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) in 238 admitted hospital patients, Lei Liu1 et. al. https://www.medrxiv.org/content/10.1101/2020.03.06.20031856v1
2. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019?, Juanjuan Zhao et al., The Lancet Mar 2020 https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3546052
3. Food and Drug Association (FDA)
4. The role of immunochemical rapid tests for the determination of antigens in diagnosing infection caused by the SARS-Cov-2 virus, WHO, Recommendations of September 11, 2020. https://apps.who.int/iris/bitstream/handle/10665/334253/WHO-2019-nCoV-Antigen Detection-2020.1-rus.pdf?sequence=18&isAllowed=y
5. Standards of medical care "CORONAVIRUS DISEASE (COVID-19)» https://moz.gov.ua/nakazi-moz
All necessary links for travelers to Ukraine↓
The Ministry of Health of Ukraine advises that PCR document showing results must:
Be in English (translation into Ukrainian is preferable, if possible - not obligatory)
State the sample collection time and the time when received results.
Specifically, mention the test type – PCR – polymerase chain reaction.
Show the full name, date of birth, and passport number of the traveler.
Include the name, address, and contact details of the lab performing the test
Clearly state the result – COVID-19 NO or COVID-19 YES.
COVID-19 Testing
COVID-19 tests are widely available at the expense of the individual.
The average cost of a PCR test is 60 USD.
Information Resources:
Other links:


